[ad_1]
Probably the greatest puzzle in sensory biology is magnetoreception – how animals perceive the earth’s magnetic field and use it as a compass to determine their spatial orientation. Animals as diverse as birds, sea turtles, fish, crustaceans and insects rely on this area for both short and long-range navigation1. The identity of the biological tissue responsible for sensing the direction of the field and the sensory mechanism underlying this type of navigation remain a mystery. In migratory birds, the main competitors are magnetically sensitive proteins, so-called cryptochromes, which are located in the retina2. However, there is no evidence that these proteins really have the magnetic sensitivity and physical properties needed to detect Earth’s extremely weak magnetic field. Registered mail nature, Xu et al.3 provide this evidence in vitrowhich temptingly brings us close to solving the mystery of magnetoreception.
There are currently two main hypotheses about how animals sense the earth’s magnetic field1,4th (as well as some alternative hypotheses that have been made in recent years5,6th). It is suggested that crystals of the oxidized iron compound magnetite (Fe3Ö4th) residing in his body and facing the field exert a rotational force – called torque – on mechanoreceptors with which they are in physical contact. This could thereby signal changes in body alignment through the opening and closing of mechanoreceptor ion channels.
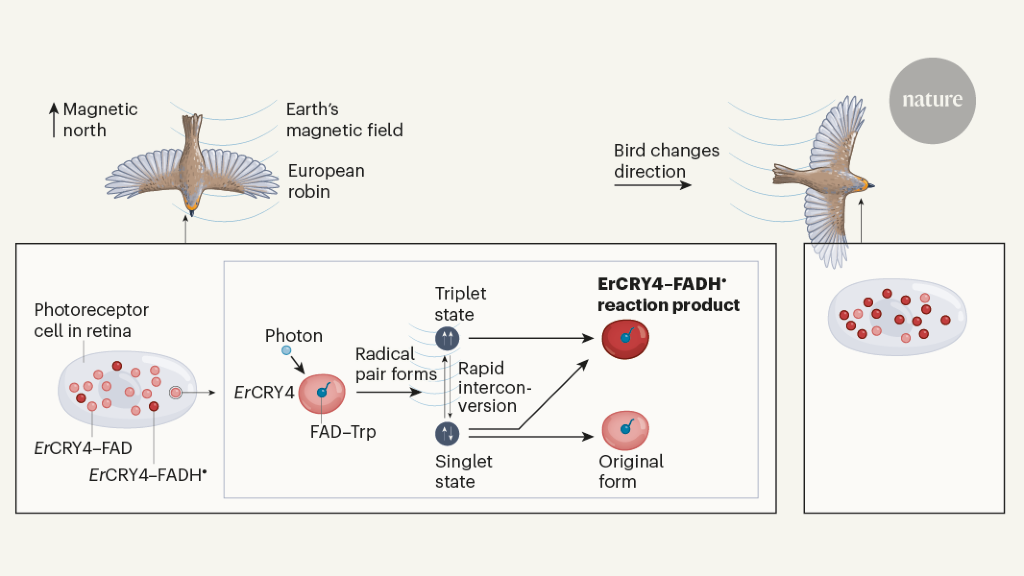
The other main hypothesis (Fig. 1) is that when cryptochrome proteins absorb light photons and are “photoexcited” they form magnetically sensitive chemical intermediates known as radical pairs. Fluctuations in the yield of their reaction product (the form of cryptochrome, which contains a radical molecule called FADH.)•) are intended to signal the direction of the animal in relation to the earth’s magnetic field7th,8th. These two proposed mechanisms are not mutually exclusive6th – Migratory birds could indeed have both magnetite for their “magnetic card” sense (the ability to have magnetic properties associated with a particular location on the earth’s surface) and cryptochromes for their magnetic compass sense (which provides a path for the animal) to determine its direction relative to magnetic north)1,2.
Found in both animals and plants, cryptochromes are a type of protein known as a flavoprotein. Cryptochromes bind non-covalently to a molecule called a chromophore, like FAD, which in its fully oxidized state absorbs photons from blue light. In animals, the cryptochrome proteins CRY1 and CRY2 are involved in the regulation of the daily (circadian) rhythm9, and their tissue expression levels are typically cycled for 24 hours. In contrast, the cryptochrome CRY4 lacks such signs of a circadian cycle, suggesting that it plays a different biological role, possibly that of magnetoreception10,11. CRY4 occurs only in birds, fish, and amphibians, which are species with well-documented magnetically guided behaviors. CRY4 has therefore emerged as the leading candidate for enabling cryptochrome-based magnetoreception in vertebrates.
Previous analysis10 of chickens (Gallus gallus) and migratory robins (Erithacus rubecula) indicates that CRY4 is located in the outer segments of two types of photoreceptor cells in the retina – double cones and long-wave single cones. This is an ideal place to receive the light that excites cryptochromes and thus supports magnetic perception. Further evidence, consistent with a possible role for CRY4 in magnetoreception, is that its level of expression in the robin’s retina increases as the migration season approaches, while its level remains persistently low in non-migratory chickens10.
The greatest advance by Xu and his colleagues is the demonstration that the version of CRY4 (dubbed HeCRY4) in the migratory robin has a crucial property that is needed to detect the earth’s magnetic field: the ability to form radical pairs with high magnetic sensitivity. Radical pairs arise when the FAD is on HeCRY4 is reduced (receives an electron) in the presence of light. Radicals contain an odd number of electrons, and a radical pair consists of two radicals formed at the same time, usually through a chemical reaction. in the HeCRY4, the odd electrons of the radicals, are generated by sequential electron hopping along a chain of three or four tryptophan amino acid residues (referred to as TrpA according to TrpD.), which are located between the FAD and the surface of the cryptochrome.
In the case of FAD, the odd electron produced in the reduction in the presence of light makes the radicals intrinsically magnetic. This is because electrons behave like microscopic magnets, with a property physicists refer to as spin (typically symbolized by an arrow ↑). In a molecule with an even number of electrons, the spins of each electron pair cancel each other out, making the molecule non-magnetic.
If the spins of the odd electrons in each of the two radicals in a radical pair are antiparallel (↓ ↑), the radical pair adopts a singlet state, but if they are parallel (↑↑), the pair occupies a triplet state. When cryptochrome is photoexcited it always forms a radical pair in the singlet state, but it doesn’t stay that way for long. Due to a peculiarity of quantum mechanics, the radical pair quickly converts to the triplet state and then jumps back and forth between these two states millions of times per second. Either of these two states can produce a reaction product – the form of CRY4, which contains the radical FADH. contains•, the proposed signaling molecule for magnetoreception (Fig. 1). The singlet state can, however, also return to its oxidized, unexcited ground state, whereby its relative contribution to the formation of reaction products is reduced. So if the conversion of the singlet and triplet states into one another can be manipulated to change the relative time spent in each of the two states, the yield of the reaction products can also be manipulated, since a greater proportion of time in the triplet state results in a higher yield of reaction products.
This is the heart of the proposed magnetosensor based on cryptochrome: The relative residence time in the singlet and triplet state and the yield of reaction products are directly manipulated by the direction of the earth’s magnetic field. The interplay between an individual HeCRY4 molecule and the field alone is at least a million times too weak to generate the radicals and affect their stability2, but the required energy is provided by the photon absorbed by FAD. However, for this to work at all, the radical pair must be sufficiently sensitive to magnetic fields and the reaction product must exist long enough and have a sufficiently high yield to realistically act as a sensory signal substance. In a tour de force of biophysical chemistry, Xu and colleagues used a wide range of techniques, such as spectroscopic methods and molecular dynamic simulations, to show that all of these conditions passed HeCRY4, at least in vitro.
Not only HeCRY4 has a much higher magnetic sensitivity than CRY4 proteins in non-migratory pigeons and chickens, but has site-specific mutations of amino acid residues in it HeCRY4 also reveals that its TrpD. is probably responsible for the generation of high and long-lasting (lasting more than a millisecond) yields of reaction products that would be required for magnetosensory signal transmission. Although the evidence presented by Xu and colleagues is not definitive evidence that HeCRY4 is the elusive magnetoreceptor in vivo, the authors have brought us closer and closer to the solution of this enduring mystery of sensory biology.
Competing interests
The author does not declare any competing interests.
[ad_2]