Nobel laureates in the field of physics include Alain Aspect, John F. Clauser, and Anton Zeilinger for their contributions to our understanding of quantum mechanics and the behavior of subatomic particles, paving the way for future developments in areas such as supercomputing and secure communications.
The study of how and why different bodies move and interact is at the heart of mechanics, a branch of physics. There are two branches of mechanics: classical and quantum.
The mathematical study of the motion of macroscopic objects and the forces acting on them is known as classical or Newtonian mechanics.
Particles, including atoms, electrons, photons, and just about everything else in the molecular and submolecular world, are at the heart of quantum mechanicsa branch of physics.
In the simplified chemistry of “click chemistry,” chemical building blocks click together easily and quickly. It is a chemical approach that reacts quickly and has few by-products, making it ideal for industrial applications.
Barry Sharplesswho used the term “Click Chemistry‘ in the early 2000s discovered that it is easier to attach whole carbon skeletons to smaller molecules than to force carbon atoms, the building blocks of organic matter, to bond to one another. One way to speed up the process while reducing waste is to use simple interactions between molecules with a “stronger selfishness“ to join together.
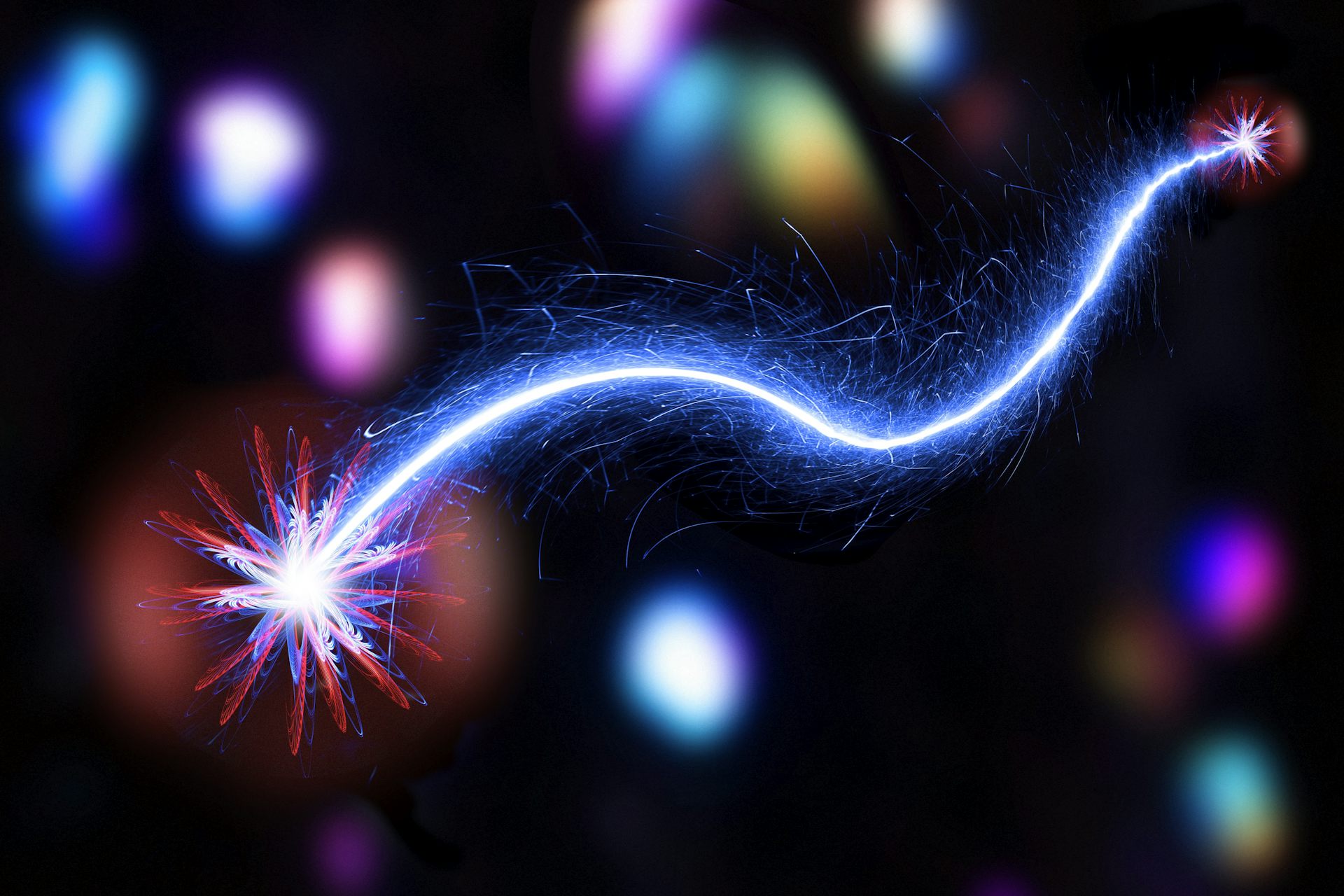
quantum entanglement

When two subatomic particles are allowed to coexist in a quantum state where their properties are complementary, and the attributes of one particle can be measured to reveal the properties of the other particle, this is called quantum entanglement.
This is true for any distance the two particles are moved apart.
Erwin Schrödinger’s 1935 explanation of quantum entanglement and the famous “cat dilemma‘ that has been produced are both his contributions to the field.
It is the phenomenon that occurs in physics when two or more particles are created in a way that makes it impossible to characterize the quantum state of a single particle in a pair or group separately from the state of the others.
Due to the nature of quantum mechanics, it is necessary to explain the quantum states of several objects in relation to one another, even if they are spatially far away.
This enables us to see connections between the measurable physical properties of the systems.
Einstein thought this was a “spooky activity‘ which was his way of saying it was a bad idea.
Bell inequality
A mathematical inequality called Bell inequality was developed by John Stewart Bell in the 1960s. It claims so If there are hidden variables, the correlation between the results of multiple measurements would never be greater than a certain threshold.
A violation of Bell’s inequality, leading to a larger correlation than would normally be achievable, is predicted by quantum mechanics for a particular class of experiments.
meaning
With the development of experimental instruments, the basis for a new technology age based on quantum information was created.
It helps in the development of quantum computerthe refinement of measurement techniques, the construction of quantum networks, the establishment of secure quantum-encrypted communication (quantum cryptography) and the observance of precise timing, as is the case with atomic clocks.
Bioorthogonal reactions (Bertozzi):
These processes can be carried out within living creatures without affecting the cell’s natural chemistry. Molecular bioimaging, targeted delivery, in situ drug activation, investigation of cell-nanomaterial interactions, biosensingetc. all benefit from integration with nanotechnology. Researchers have improved the targeting of cancer drugs by using bioorthogonal processes.

The development of Bertozzi’s cancer-fighting click chemistry and how he achieved it.
recognize glycans: Carolyn R. Bertozzi, who studied glycans, a cryptic carbohydrate present on cell surfaces and vital to the immune system, hoped so improve the visibility of glycans by attaching fluorescent molecules to them.
Bertozzi chose to use azide, the same chemical used by Sharpless and Meldal. Not only is the azide non-toxic, it also does not react with other components of the cell.
in 2004, She found a way to perform the same click chemistry reaction without using the harmful metal copper.
Bertozzi’s work is used to detect glycans on the surface of tumor cells and thus switch off their defense systems.
People with advanced cancer are the focus of current clinical trials of this approach.
Clickable antibodies are also being developed to aid in tumor tracking and radiation targeting cancer cells.